Cartilage Regeneration in Human with Adipose Tissue-Derived Stem Cells: Current Status in Clinical Implications
Jaewoo Pak 1, Jung Hun Lee 2, Wiwi Andralia Kartolo 3, Sang Hee Lee 4
1 Stems Medical Clinic, 32-3 Chungdam-dong, Gangnam-gu, Seoul 06068, Republic of Korea.
2 Stems Medical Clinic, 32-3 Chungdam-dong, Gangnam-gu, Seoul 06068, Republic of Korea; National Leading Research Laboratory, Department of Biological Sciences, Myongji University, 116 Myongjiro, Yongin, Gyeonggido 17058, Republic of Korea.
3 FMN Wellness & Antiaging Centre, Jalan Sangihe 15A, Jakarta Pusat 10150, Indonesia.
4 National Leading Research Laboratory, Department of Biological Sciences, Myongji University, 116 Myongjiro, Yongin, Gyeonggido 17058, Republic of Korea.
ABSTRACT
Osteoarthritis (OA) is one of the most common debilitating disorders among the elderly population. At present, there is no definite cure for the underlying causes of OA. However, adipose tissue-derived stem cells (ADSCs) in the form of stromal vascular fraction (SVF) may offer an alternative at this time. ADSCs are one type of mesenchymal stem cells that have been utilized and have demonstrated an ability to regenerate cartilage. ADSCs have been shown to regenerate cartilage in a variety of animal models also. Non-culture-expanded ADSCs, in the form of SVF along with platelet rich plasma (PRP), have recently been used in humans to treat OA and other cartilage abnormalities. These ADSCs have demonstrated effectiveness without any serious side effects. However, due to regulatory issues, only ADSCs in the form of SVF are currently allowed for clinical uses in humans. Culture-expanded ADSCs, although more convenient, require clinical trials for a regulatory approval prior to uses in clinical settings. Here we present a systematic review of currently available clinical studies involving ADSCs in the form of SVF and in the culture-expanded form, with or without PRP, highlighting the clinical effectiveness and safety in treating OA.
Introduction
Osteoarthritis (OA) is a common painful and debilitating disorder in the elderly [1, 2]. All current medical treatments for OA, such as nonsteroidal anti-inflammatory drugs (NSAIDs), steroids, and hyaluronic acids (HAs), physical therapy, aim to remedy the symptoms, as opposed to treating the underlying causes. When failed with symptomatic medical treatments, patients usually resort to receiving total knee replacement (TKR) or total hip replacement (THR) surgery.
Method
We used the Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA) in our review (Figure 1) [31]. We conducted a systematic literature search in PubMed, Medline, and Embase. We used the keywords as our search terms. We combined terms for selected indications (stem cell, osteoarthritis, and adipose). The literature search included all studies published in English between 2000 and 2015. We identified 253 references after removing duplicates. We independently assessed full-text articles for inclusion in our review. The criteria for the inclusion of studies in our review encompassed clinical studies on ADSC injection conducted on humans for cartilage regeneration. Finally, we found 13 articles showing clinical studies on ADSC treatments for cartilage defects (Figure 1).
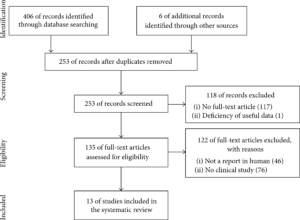
(Figure 1) Literature selection process (PRISMA flow diagram).
Discussions
Adipose tissue is considered to be a preferable source of MSCs due to its ease of accessibility and the availability of a large number of stem cells per gram of adipose tissue. In adipose tissue, 1% to 10% of nucleated cells are considered to be ADSCs whereas only 0.0001–0.01% of nucleated cells in the bone marrow are stem cells [14]. In addition, the number of nucleated cells in adipose SVF can range from 500,000 to 2,000,000 cells per gram of adipose tissue [40]. The range of MSCs in 1g of adipose tissue may be 5,000–200,000 stem cells [40]. Thus, theoretically, 0.5–20 million ADSCs can be extracted from 100g of adipose tissue. If the number of MSCs in adipose SVF is 5%, approximately 10 million ADSCs can be obtained from 100g of adipose tissue.
Conclusions
At present, there is no cure for painful OA in stages 2 and 3. For these patients, the intra-articular injection of ADSCs in the form of SVF can be an alternative treatment for now. As described in this review, the joint injection of ADSCs in the form of SVF with PRP can be safe and efficacious. Moreover, obtaining approximately 100 g of adipose tissue and percutaneous joint injections is considered to be a minimally invasive procedure and can be readily accepted by patients. These procedures carry relatively low rates of morbidity and side effects.
Although a large amount of injecting ADSCs is more efficacious in regenerating cartilage, the studies reviewed in this paper have shown that ADSCs in the form of SVF with PRP can be efficacious in symptom improvement.
However, lack of well-designed studies with control on using different methods and components of the injections still leaves many questions unanswered. In addition, the lack of understanding of the mechanism of action of ADSCs dictates the need for more clinical trials.
PMID: 26881220 PMCID: PMC4736810
To read the full article, click here, DOI: 10.1155/2016/4702674
Related: adipose-derived stromal/stem cells, adipose tissue, Osteoarthritis, stromal vascular fraction, stem cells population, regenerate cartilage, cartilage regeneration

